The Center for Chemical Evolution is searching for molecules and reactions responsible for the initial synthesis and evolution of the polymers associated with life. This question poses a number of intellectual and technical challenges. Researchers within the CCE hypothesize that the first biopolymers resembled those known in life today but had unique properties that allowed them to survive, flourish, and evolve on the early Earth. These proto-biopolymers and the environments that fostered their survival define the research themes within the CCE.
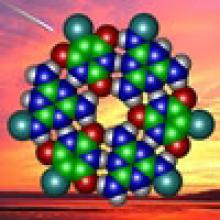 Proto-Nucleic Acids
Proto-Nucleic Acids
The RNA World hypothesis, which suggests early life relied on RNA’s ability to store genetic information as well as perform catalytic functions to exist, is central to many current theories on the evolution of life. Based on plausibly prebiotic conditions, there were likely formidable challenges to the formation of RNA on the early Earth. The Proto-Nucleic Acids theme is considering plausibly prebiotic scenarios resulting in the formation of RNA or a precursor molecule. Studies of the various components (e.g., base, sugar, backbone) have revealed alternatives to the Watson-Crick bases and the phosphodiester backbone that may result in the formation of biopolymers with similar structural and genetic characteristics to extant nucleic acids. The following sections highlight recent discoveries.
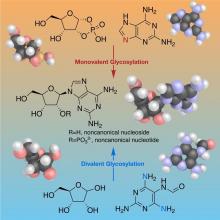 Chemistry of Nucleoside Formation
Chemistry of Nucleoside Formation
The RNA World hypothesis, which suggests early life relied on RNA’s ability to store genetic information as well as perform catalytic functions to exist, is central to many current theories on the evolution of life. Based on plausibly prebiotic conditions, there were likely formidable challenges to the formation of RNA on the early Earth. The Proto-Nucleic Acids theme is considering plausibly prebiotic scenarios resulting in the formation of RNA or a precursor molecule. Studies of the various components (e.g., base, sugar, backbone) have revealed alternatives to the Watson-Crick bases and the phosphodiester backbone that may result in the formation of biopolymers with similar structural and genetic characteristics to extant nucleic acids. The following sections highlight recent discoveries.
 The Origins of Chirality in Biopolymers
The Origins of Chirality in Biopolymers
The RNA World hypothesis, which suggests early life relied on RNA’s ability to store genetic information as well as perform catalytic functions to exist, is central to many current theories on the evolution of life. Based on plausibly prebiotic conditions, there were likely formidable challenges to the formation of RNA on the early Earth. The Proto-Nucleic Acids theme is considering plausibly prebiotic scenarios resulting in the formation of RNA or a precursor molecule. Studies of the various components (e.g., base, sugar, backbone) have revealed alternatives to the Watson-Crick bases and the phosphodiester backbone that may result in the formation of biopolymers with similar structural and genetic characteristics to extant nucleic acids. The following sections highlight recent discoveries.
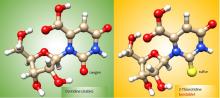 Oxygen vs. Sulfur: Battle for Stability
Oxygen vs. Sulfur: Battle for Stability
The RNA World hypothesis, which suggests early life relied on RNA’s ability to store genetic information as well as perform catalytic functions to exist, is central to many current theories on the evolution of life. Based on plausibly prebiotic conditions, there were likely formidable challenges to the formation of RNA on the early Earth. The Proto-Nucleic Acids theme is considering plausibly prebiotic scenarios resulting in the formation of RNA or a precursor molecule. Studies of the various components (e.g., base, sugar, backbone) have revealed alternatives to the Watson-Crick bases and the phosphodiester backbone that may result in the formation of biopolymers with similar structural and genetic characteristics to extant nucleic acids. The following sections highlight recent discoveries.


